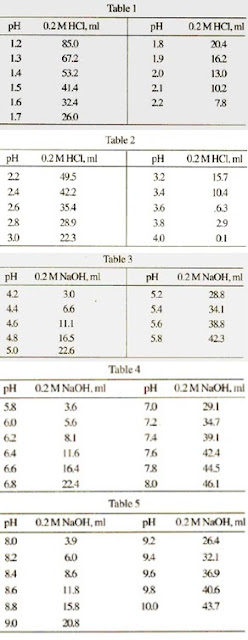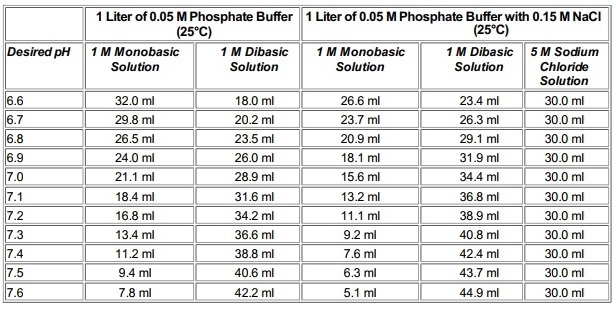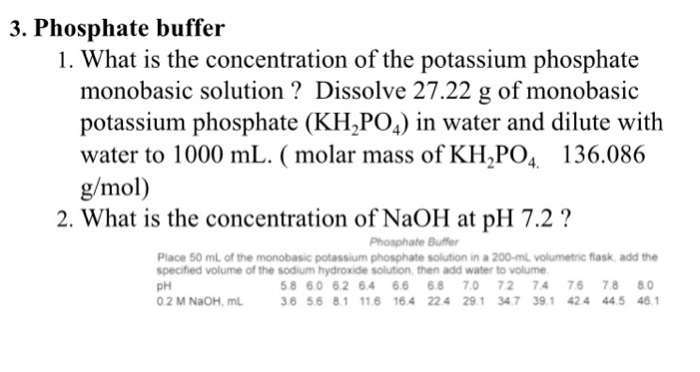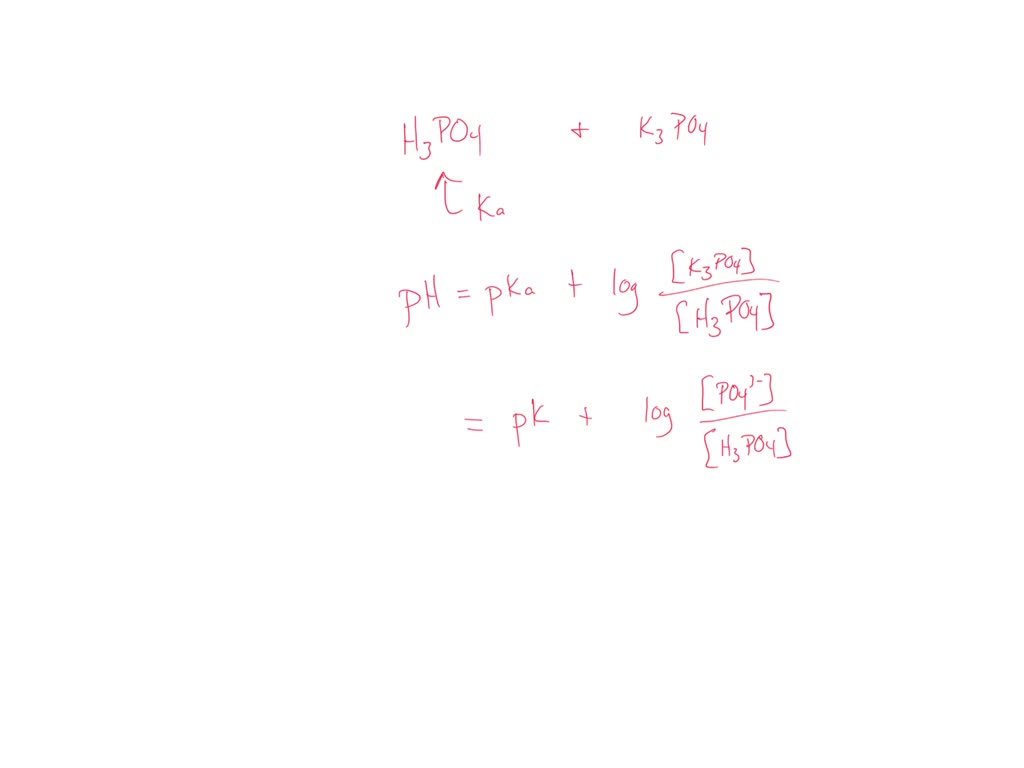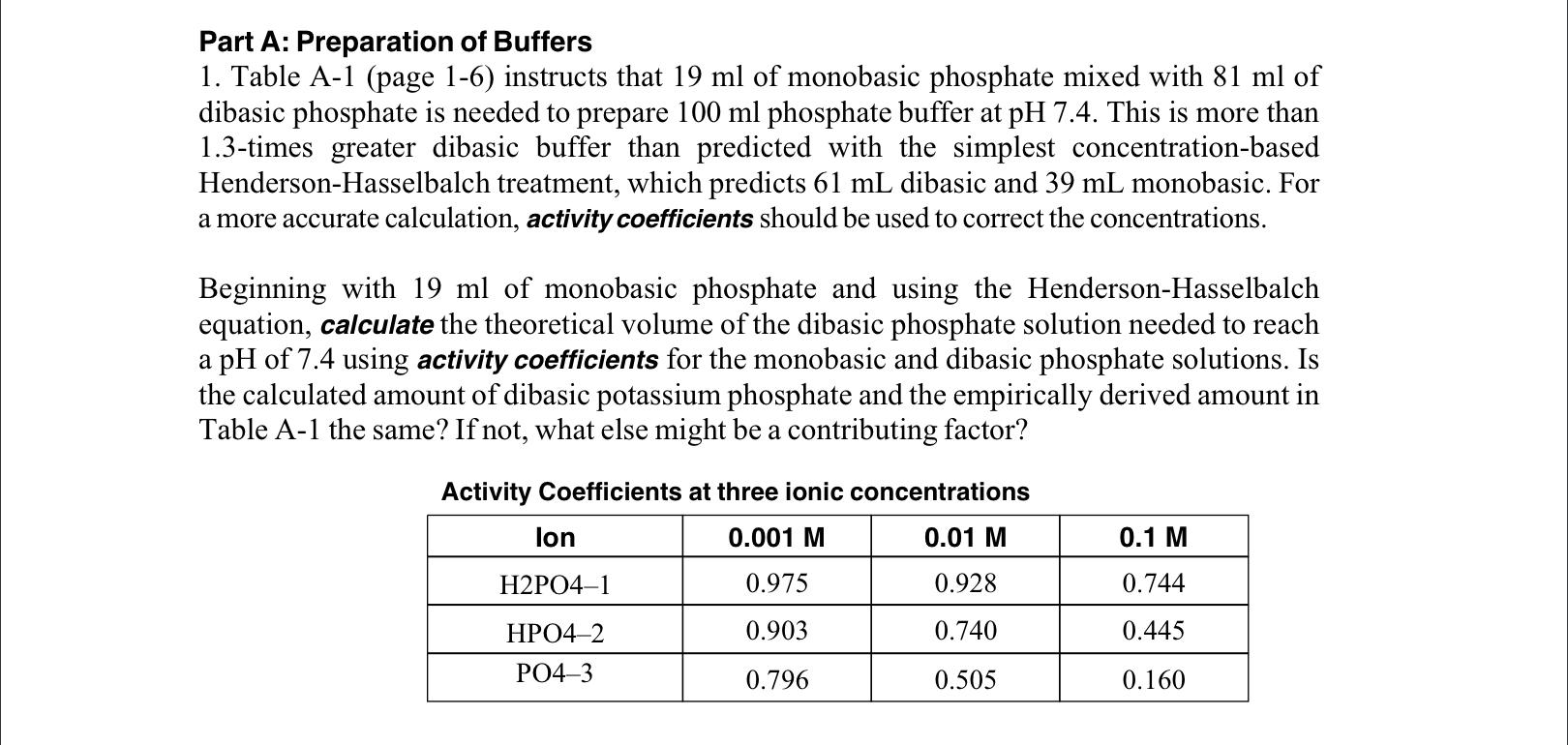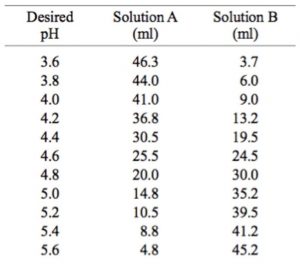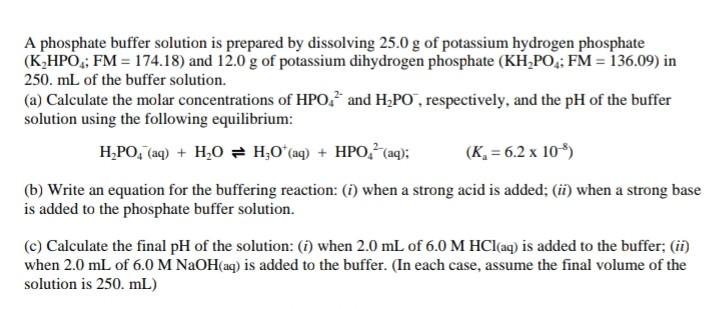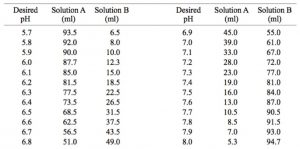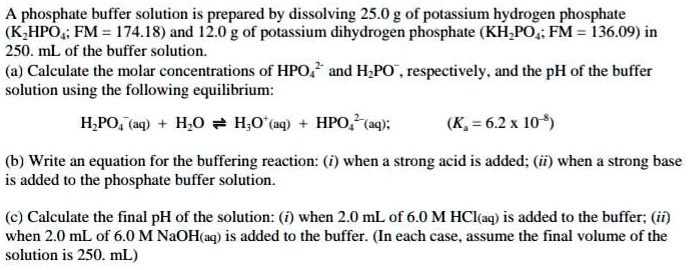
SOLVED: A phosphate buffer solution is prepared by dissolving 25.0 g of potassium hydrogen phosphate (K2HPO4; FM = 174.18) and 12.0 g of potassium dihydrogen phosphate (KH2PO4; FM = 136.09) in 250
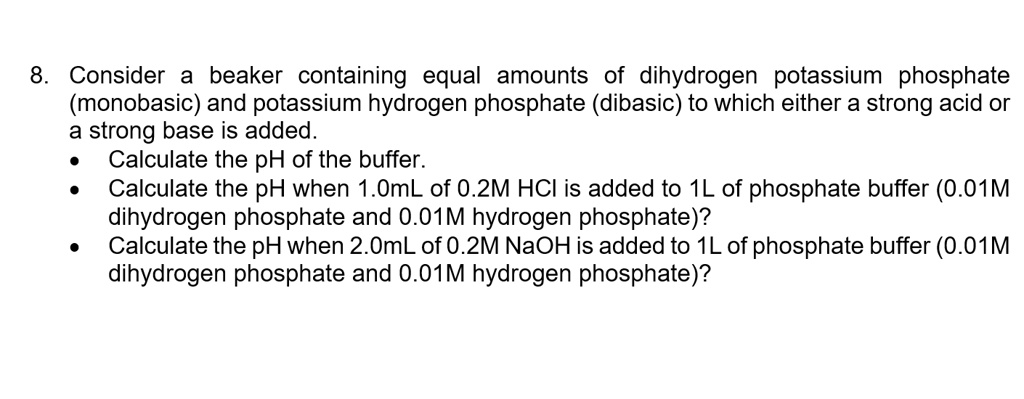
SOLVED: 8. Consider a beaker containing equal amounts of dihydrogen potassium phosphate (monobasic) and potassium hydrogen phosphate (dibasic) to which either a strong acid or strong base is added. Calculate the pH

Concentrations of potassium phosphate buffer and rham- nolipid investigated | Download Scientific Diagram

velocities were obtained in potassium phosphate buffer at 30 mC, pH... | Download Scientific Diagram

Mobile Phase Buffers in Liquid Chromatography (LC): Effect of Buffer Preparation Method on Retention Repeatability